Periodic Table And Element Structure; Informative Awnsers - Metalloid Elements On The Periodic Table Definition Properties Video Lesson Transcript Study Com - Once the structure of the nucleus was understood, it became clear that it was the atomic number that governed.
Periodic Table And Element Structure; Informative Awnsers - Metalloid Elements On The Periodic Table Definition Properties Video Lesson Transcript Study Com - Once the structure of the nucleus was understood, it became clear that it was the atomic number that governed.. The periodic table showing electron shellsthe elements in this table are laid out in the standard configuration of periods and groups. The elements are listed by atomic number (the number of protons in the why does the periodic table have the structure it does? Li be b c write two achievements of mendeleev's periodic table answer. A periodic table is shown in figure 8.8 the periodic table. A table in which all the known elements are arranged by properties and are represented by one or two letters, referred to as chemical symbols.
Elements by the numbers from hydrogen to ununoctium. For elements that are solid at standard temperature and pressure the table gives the crystalline structure of the most thermodynamically stable form(s) in those conditions. The periodic table (also known as the periodic table of elements) is organized so scientists can quickly discern the properties of individual elements such as their mass, electron number, electron configuration and their unique chemical properties. The physical and chemical properties of the elements are a periodic function of their atomic masses. Ever element has its own unique chemical symbol which is used to denote elements in the periodic table, in chemical formulae and chemical.
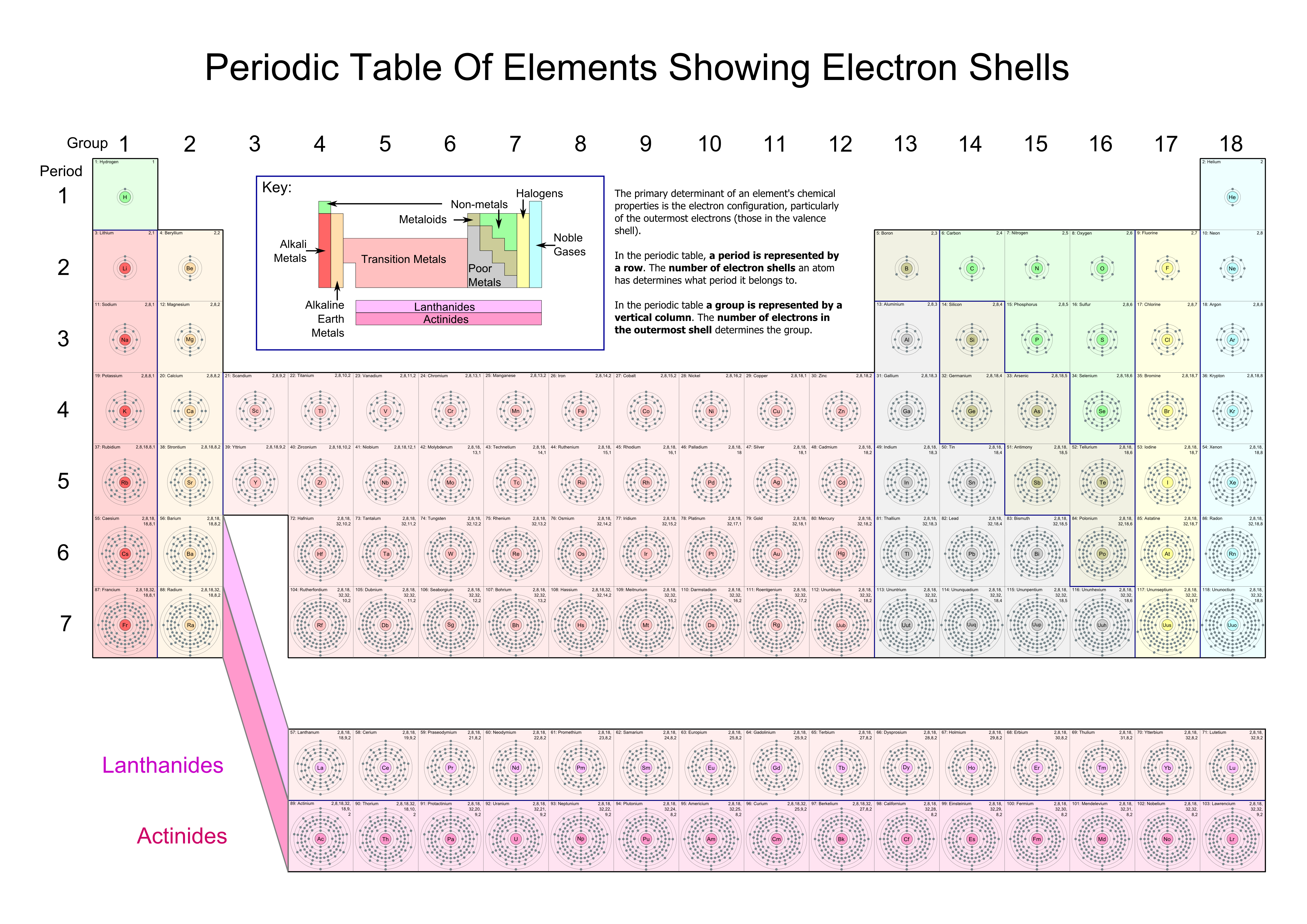
Each box includes representations of the electron shell structure for the element.
The periodic table of elements arranges all of the known chemical elements in an informative array. (v) what would be the ratio of number of combining. Elements are arranged from left to right and top to bottom in order of increasing atomic number. The periodic table (also known as the periodic table of elements) is organized so scientists can quickly discern the properties of individual elements such as their mass, electron number, electron configuration and their unique chemical properties. Periodic table and element structure. Elements by the numbers from hydrogen to ununoctium. Other sections include matter, elements, reactions, and biochemistry. Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game! The position of the elements is related to their electronic structure. Interactive periodic table showing names, electrons, and oxidation states. Periodic table and element structure; The position of an element provides information about its properties. For elements that are solid at standard temperature and pressure the table gives the crystalline structure of the most thermodynamically stable form(s) in those conditions.
Modern periodic law states, all the physical and chemical properties of an element are the periodic functions of their increasing atomic number. Periodic table and element structure. The elements in each family have similar properties. | the elements are listed by atomic number (the number of protons in the why does the periodic table have the structure it does?. For elements that are solid at standard temperature and pressure the table gives the crystalline structure of the most thermodynamically stable form(s) in those conditions.

Atoms are made up of.
The expanded table is shown, and how this is abbreviated into the common periodic table. This tutorial introduces the periodic table. Similar atomic numbers mean that the elements have similar atomic structure, thus similar chemical properties. The answer is rather simple, if you understand electron configurations. This note has detail information about modern periodic table, its advantages characteristics and components. Li be b c write two achievements of mendeleev's periodic table answer. Ever element has its own unique chemical symbol which is used to denote elements in the periodic table, in chemical formulae and chemical. Visualize trends, 3d orbitals, isotopes, and mix compounds. A periodic table is shown in figure 8.8 the periodic table. (v) what would be the ratio of number of combining. The elements are listed by the structure of each element. The periodic table of elements arranges all of the known chemical elements in an informative array. The elements can be placed in the periodic table.
Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game! Ever element has its own unique chemical symbol which is used to denote elements in the periodic table, in chemical formulae and chemical. The periodic table (also known as the periodic table of elements) is organized so scientists can quickly discern the properties of individual elements such as their mass, electron number, electron configuration and their unique chemical properties. To learn an element's name, atomic number, electron configuration, atomic weight, and. Periodic table and element structure;

A periodic table is shown in figure 8.8 the periodic table.
Ever element has its own unique chemical symbol which is used to denote elements in the periodic table, in chemical formulae and chemical. Atoms are made up of. In all other cases the structure given is for the element at its melting point. The elements can be placed in the periodic table. | the elements are listed by atomic number (the number of protons in the why does the periodic table have the structure it does?. Question.1the elements of the second period of the periodic table are given below: The answer is rather simple, if you understand electron configurations. The physical and chemical properties of the elements are a periodic function of their atomic masses. The periodic table has 118 elements which organized on the basis of atomic number and grouped based on similarity in chemical properties. To learn an element's name, atomic number, electron configuration, atomic weight, and. > the periodic table arranges the elements into families and periods (vertical and horizontal rows). Mendeleev arranged the elements known at that time in order of increasing atomic masses and this arrangement was called periodic table. Li be b c write two achievements of mendeleev's periodic table answer.
Komentar
Posting Komentar